


The chicken and the egg…
This article has over 25 recent peer-reviewed scholarly references
The gut–brain axis is a bidirectional link, and which facilitates communication between the brain and the gut, it has been discovered that gut dysbiosis can alter brain function and trigger the development of mental illness disorders such as anxiety, Parkinson’s (Arneth, 2018), depression (Capuco et al, 2020), Alzheimer’s (Fujii et al., 2019), and autism (Srikantha & Mohajeri, 2019; Pulikkan et al., 2018; Masi et al, 2017).
Despite considerable progress, researchers are only beginning to explore the numerous neuro modulatory signals and structures that comprise the gut microbiota–brain axis (Bauer et al., 2016). The axis has expanded recently to include the entire central nervous system (not just the brain) (Wang and Kasper, 2014; Bauer et al., 2016).
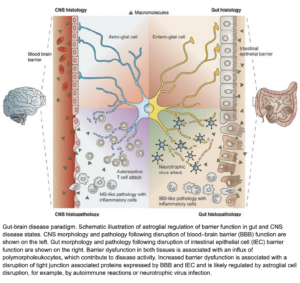 (Savidge et al, 2007)
(Savidge et al, 2007)This picture demonstrates the catastrophic events that can occur when we have reduced tight junction action in both the Gut and the Blood brain barrier (BBB), but also shows how the nervous system becomes under attack when our lining is compromised in the brain, like how our immune system becomes under attack when our lining is compromised in the gut. This leads to inflammation and inflammatory markers be sent to and from the brain and gut (dis-regulated immune response)– this is not helpful when this occurs on a chronic timeline.
Leaky Gut – Leaky Brain barrier can lead to some of the worst-case scenarios and unfortunate common diagnosis like Parkinson’s and Alzheimer’s. It was found in a recent study that children with Autism spectrum disorder showed altered tight junctions and increase permeability in both the brain and the gut (Srikantha & Mohajeri, 2019).
YES > there is massive amounts of communication between the Gut and the Brain via chemical and metabolic signals as well as via the Vagus Nerve…
This cross talk allows the brain to signal the gut and vice versa. Researchers have reported that the microbiota has the ability to influence enteric nervous system (Gut nerves) activity through the production of unique molecules (cytokines and hormones (Masi et al., 2017)) with the ability to alter the function of various neurotransmitters such as γ-aminobutyric acid (GABA), melatonin, histamine, serotonin and acetylcholine (Sampson & Mazmanian, 2015).
Mediators affect all aspects of brain physiology, including feeding behavior, sleep, thermoregulation, emotion, and memory, and their interactions with the BBB are an integral part of the cytokine network (Masi et al., 2017; Pan et al., 2011). These mediators are involved in the central nervous system signaling, and are also involved in metabolic and endocrine system signalling, which is why they are associated with neuro-psychiatric disorders such as autism, depression, anxiety, stress (Wang and Kasper, 2014). Chronic cytokine release has been linked to disrupted epithelial lining integrity and influencing the transport of brain mediators – as cytokines can enter the BBB.
In other words: your microbiota are responsible for creating mediators or signals, which can change how your neurotransmitters, metabolic and endocrine systems function.
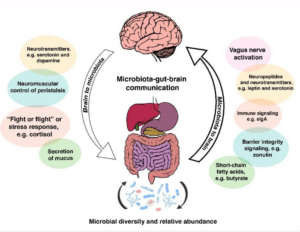
They also communicate via the vagus nerve:
Through the vagus nerve (actually two right and left), the intestinal microbiota can influence different processes such as the production of metabolic precursors, neurotransmitters and active metabolites (Sampson & Mazmanian, 2015).
To me this clearly suggests if our gut microbiota are under stress and potentially imbalanced, they might not be sending all the right signals to these VITAL systems in our body and our brains.
Many of the articles I read suggest those people suffering from mental illness often have gastrointestinal issues and vice versa, this is not a coincidence! One study shows similar states of dysbiosis (dis-balance of the gut microbiota) found in patients with depression disorder shows the link and the bidirectional communication, and indicates the effects of the microbiome on the hypothalamic – pituitary stress response (Schneiderhan et al., 2016)
It’s a two-way street!
It is important to note – while our microbiome is responsible for sending signals to our brain – our behavior and cognitive function of the brain can create imbalance in the microbiome as well (Rogers et al., 2016)
Serotonin is a hormone that stabilizes our mood and is responsible for numerous vital body functions. 90% of serotonin is localized in the epithelial gut cells – and requires specific microbiota to be synthesized – so it can carry out its ‘good’ feeling jobs (see list below).
The microbiota (of course) release metabolites including Short Chain Fatty Acids (SCFA’s) which stimulates the epithelial cells to increase expression of the amino acid tryptophan subsequently niacin – which is required for serotonin synthesis (Rode et al., 2020).
“Recent data also shows that bacterial products such as short chain fatty acids can upregulate serotonin production by the enterochromaffin cells” (Reigstad et al, 2015 as cited in Jenkins et al., 2016, 10.1)
Bacteria known to digest tryptophan include: Actinobacteria, Firmicutes, Bacteroidetes, Proteobacteria, and Fusobacteria (Jenkins et al., 2016). Further, five genera, namely, Clostridium, Burkholderia, Streptomyces, Pseudomonas, and Bacillus have been predicted to be enriched tryptophan metabolism pathways, suggesting a higher potential of these bacterial groups to metabolize tryptophan in gut (Kaur et al., 2019).
The decreased diversity and stability of the gut microbiota may dictate serotonin-related health problems in the elderly (Berger et al., 2009).
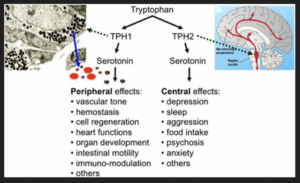
FYI: Serotonin regulates numerous biological processes including cardiovascular function, bowel motility, ejaculatory latency, and bladder control. Additionally, new work suggests that serotonin may regulate some processes, including platelet aggregation, by receptor-independent, transglutaminase-dependent covalent linkage to cellular proteins (Berger et al., 2009).
These tissue healing molecules are made when our healthy and diverse microbiota eat and metabolize certain foods (Acetate, propionate, and butyrate) – insoluble fibers and resistant starches to be exact. They have remarkable positive impacts on the body, immune system and the brain:
 (Riviere et al., 2016).
(Riviere et al., 2016).
Sugar and alcohol
There is clear evidence that processed substances like gluten, alcohol and sugars, and medications like antobiotics can be associated with dysbiosis and leaky gut (increased permeability of the intestinal wall) by elevating toxins and allowing over growth of opportunistic resident microbiota to create imbalance (Carlson et al., 2016).
Start with a few easy stress reducing activities like drawing, yoga and meditation. Incorporate them into your daily routine- believe me you will see results. Also, carve out time to relax- read a book, make a new healthy recipe or just have a cat nap. This will work in the top down effect, promoting a reduction in CNS stress response mediator release (eg. cytokines, cortisol) that can lead to disruptions in the Gut lining via the Gut-brain axis.
Because they turn into SCFA’s! The more prebiotics (dietary fibres) you consume and the more diverse your microbiome will be, and the greater chance you have of feeding the right bacteria which converts this fibre into SCFA’s.Try to incorporate leeks, asparagus, artichoke, garlic and onions (there are many more) into your diet on a regular basis. They also aid in the conversion of tryptophan to serotonin.
Tryptophan (an essential amino acid- not made in the body) is needed for serotonin synthesis in the gut (where over 90% is made). Unfortunately when you consume protein rich foods (high tryptophan foods), branched chain amino acids (BCAAs) leucine, isoleucine and valine usually compete for binding sites in the brain – so tryptophan doesn’t get converted to serotonin to be used centrally (Jenkins et al., 2016).
A balanced gut is more effective that consuming a ‘diet’ high in tryptophan. Which can be taken in a probiotic supplement or be ingested via fermented foods.
Whole plant foods!
There is still debate that supplements containing antioxidants actually benefit health- BUT there is clear evidence that ingesting plant food or Phytochemicals (botanical active ingredient in PLANT food) is beneficial for your health- there are over 250,000 articles since 1991 linking phytochemicals to prevention of chronic illness including Alzheimer’s, Cancer, Cardiovascular disease and more (Yeung, 2019).
I hope you enjoyed this overview of the gut – brain axis. If you have more questions please subscribe to my WhatsApp Wellness to receive current evidenced- based education on all things Health and Wellness!
Your Health and Wellness Specialist,
Kaley
BN MN CNC
References:
Arneth, B. M. (2018). Gut–brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function. Postgraduate medical journal, 94(1114), 446-452.
Bauer, K. C., Huus, K. E., & Finlay, B. B. (2016). Microbes and the mind: emerging hallmarks of the gut microbiota–brain axis. Cellular microbiology, 18(5), 632-644.
Berger, M., Gray, J. A., & Roth, B. L. (2009). The expanded biology of serotonin. Annual review of medicine, 60, 355-366.
Capuco, A., Urits, I., Hasoon, J., Chun, R., Gerald, B., Wang, J. K., … & Kaye, A. D. (2020). Current perspectives on gut microbiome dysbiosis and depression. Advances in Therapy, 37(4), 1328-1346.
Carlson, J., & Slavin, J. (2016). Health benefits of fibre, prebiotics and probiotics: A review of intestinal health and related health claims. Quality Assurance and Safety of Crops & Foods,8(4), 539-554.
Fujii, Y., Khasnobish, A., & Morita, H. (2019). Relationship between Alzheimer’s Disease and the Human Microbiome. In T. Wisniewski (Ed.), Alzheimer’s Disease. Codon Publications.
Gevezova, M., Sarafian, V., Anderson, G., & Maes, M. (2020). Inflammation and Mitochondrial Dysfunction in Autism Spectrum Disorder. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders)
Jenkins, T. A., Nguyen, J. C., Polglaze, K. E., & Bertrand, P. P. (2016). Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients, 8(1), 56.
Kaur, H., Bose, C., & Mande, S. S. (2019). Tryptophan metabolism by gut microbiome and gut-brain-axis: An in silico Analysis. Frontiers in neuroscience, 13, 1365.
Masi, A., Glozier, N., Dale, R., & Guastella, A. J. (2017). The immune system, cytokines, and biomarkers in autism spectrum disorder. Neuroscience bulletin, 33(2), 194-204.
Miller, I. (2018). The gut–brain axis: historical reflections. Microbial ecology in health and disease, 29(2), 1542921.
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., & Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural brain research, 277, 32-48.
Pan, W., Stone, K. P., Hsuchou, H., Manda, V. K., Zhang, Y., & Kastin, A. J. (2011). Cytokine signaling modulates blood-brain barrier function. Current pharmaceutical design, 17(33), 3729–3740.
Srikantha, P., & Mohajeri, M. H. (2019). The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. International journal of molecular sciences, 20(9), 2115.
Pulikkan, J., Maji, A., Dhakan, D. B., Saxena, R., Mohan, B., Anto, M. M., … & Sharma, V. K. (2018). Gut microbial dysbiosis in Indian children with autism spectrum disorders. Microbial ecology, 76(4), 1102-1114.
Rivière, A., Selak, M., Lantin, D., Leroy, F., & De Vuyst, L. (2016). Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Frontiers in microbiology, 7, 979. https://doi.org/10.3389/fmicb.2016.00979
Rode, J., Yang, L., König, J., Hutchinson, A. N., Wall, R., Venizelos, N., … & Vumma, R. (2020). Butyrate Rescues Oxidative Stress-Induced Transport Deficits of Tryptophan: Potential Implication in Affective or Gut-Brain Axis Disorders. Neuropsychobiology, 1-11.
Rogers, G. B., Keating, D. J., Young, R. L., Wong, M. L., Licinio, J., & Wesselingh, S. (2016). From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Molecular psychiatry, 21(6), 738-748.
Sampson, T. R., & Mazmanian, S. K. (2015). Control of brain development, function, and behavior by the microbiome. Cell host & microbe, 17(5), 565-576.
Savidge, T. C., Sofroniew, M. V., & Neunlist, M. (2007). Starring roles for astroglia in barrier pathologies of gut and brain. Laboratory investigation; a journal of technical methods and pathology, 87(8), 731–736. https://doi.org/10.1038/labinvest.3700600
Schmidt, E. K., Torres-Espin, A., Raposo, P. J., Madsen, K. L., Kigerl, K. A., Popovich, P. G., … & Fouad, K. (2020). Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. Plos one, 15(1), e0226128.
Schneiderhan, J., Master-Hunter, T., & Locke, A. (2016). Targeting gut flora to treat and prevent disease. The Journal of family practice, 65(1), 34–38.
Stilling, R. M., van de Wouw, M., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2016). The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis?. Neurochemistry international, 99, 110-132.
Wang, Y., & Kasper, L. H. (2014). The role of microbiome in central nervous system disorders. Brain, behavior, and immunity, 38, 1-12.
Wang, W., Chen, L., Zhou, R., Wang, X., Song, L., Huang, S., Wang, G., & Xia, B. (2014). Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. Journal of clinical microbiology, 52(2), 398–406. https://doi.org/10.1128/JCM.01500-13
Yeung, A. W. K., Tzvetkov, N. T., El-Tawil, O. S., Bungǎu, S. G., Abdel-Daim, M. M., & Atanasov, A. G. (2019). Antioxidants: scientific literature landscape analysis. Oxidative medicine and cellular longevity, 2019.